Professionals
Statements & Guidelines
«BackSouth African Gastroenterology Society (SAGES) position statement on the use of Radio Frequency Ablation (RFA) for the treatment of Barrett’s Oesophagus.
Introduction
The working definition
of Barrett’s oesophagus
(BO) is displacement of
the squamo-columnar
junction proximal to the
oesophago-gastric
junction. Chronic
exposure of the
oesophagus to gastric
acid, bile and digestive
enzymes results in
mucosal injury, which
may in turn lead to
replacement of normal
squamous epi-thelium by
metaplastic columnar
epithelium. Intestinal
metaplasia (IM) is the
pre- malignant lesion
for oesophageal
adenocarcinoma.
Patients with IM
typically undergo
surveillance endoscopies
every 1 to 3 years and
multiple biopsies are
taken with a view to
detecting progression to
dysplasia or
adenocarcinoma.
Elimination of the
premalignant dysplastic
epithelium may reduce
the need for
surveillance endoscopies
and the risk of
adenocarcinoma of the
oesophagus.
Management Options
for Barrett’s Oesophagus
with Dysplasia
Patients with IM
typically undergo
endoscopic surveillance
with a view to detecting
progression to dysplasia
or adenocarcinoma. Four
quadrant biopsies are
taken every 1 to 2 cm.
In patients with
low-grade dysplasia
(LGD), endoscopic
surveillance is required
every 6 months to 1 year
and in patients with
high-grade dysplasia
(HGD), every 3 to 6
months.
Thermal therapeutic
modalities
(electrocautery, laser,
argon plasma
coagulation) have been
evaluated in patients
with and without IM and
photodynamic therapy
(PDT) in patients with
HGD.
Patients with LGD are
normally entered into
long-term surveillance
programs and ablative
therapy or surgical
resection is
occasionally
recommended.
Patients with HDG
typically undergo
oesophagectomy or
photodynamic therapy.
Continued surveillance
is occasionally more
appropriate in patients
with significant
comorbidities.
A device capable of
eradicating Barrett’s
epithelium by means of
radio frequency (RF)
energy has been
available since 2005.
Over 31,000 patients
with BO in 24 countries
have treated with RFA in
the past 4 years.
Treatment is performed
in an outpatient setting
and devices causing a
360° and a 90° thermal
injury are available.
The former is used to
treat circumferential
and larger areas of IM
whereas the latter is
used to treat smaller
areas of IM.
Patients commonly
experience chest
discomfort and
odynophagia after
ablation therapy and, in
clinical trials, these
symptoms typically
resolve in 3 to 4 days.
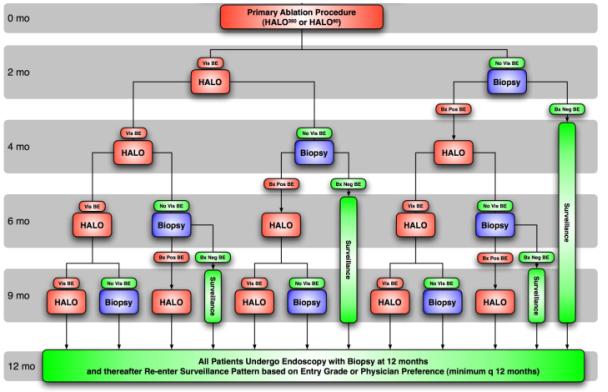
Patients are normally
reassessed 2 to 3 months
later and, should there
be residual IM,
additional ablation
therapy may be required.
Clinical studies with
the RF ablation system
have shown that complete
elimi-nation of IM is
possible in over 98 % of
patients.
Successful elimination
of the IM does not cure
the pre-existing GORD or
asso-ciated symptoms and
long-term treatment of
the GORD is therefore
still required.
Endoscopic Ablative
Therapy for Barrett’s
oesophagus
Ablation is defined as
the destruction and
ultimate removal of
diseased tissue. In the
case of BO, ablation
refers to the injury and
eradication of all IM
tissue and its
subsequent replacement
by a normal neo-squamous
epithelium. The
intention of eradicating
all IM clones and stem
cells, be it
non-dysplastic IM, LGD
or HGD, is to eliminate
or reduce the risk for
disease progression, to
reduce the risk for
cancer-related and
surgery-related death
and perhaps to reduce or
eliminate the need for
life-long surveillance.
The pre-ablation work-up
must include careful
white-light endoscopy of
the entire BO segment,
categorization of the
segment according to its
total length, the
loca-tion of its most
proximal extent, and the
location of the gastric
folds as referenced to
the incisors. Biopsies
should be obtained from
any visible abnormality,
as well as from four
quadrants from each 1 to
2 cm level of the BO.
Enhanced imaging
techniques, such as
chromoendoscopy using
Lugol’s solution, narrow
band ima-ging (NBI),
magnification endoscopy,
auto fluorescence and
high-definition
endo-scopy may increase
the detection of areas
with higher yield for
dysplasia and can-cer
and lead to more precise
staging.
Prior to consideration
for ablative therapy,
any visible focal
abnormality of the
mucosa should be
resected by means of
endoscopic mucosal
resection (EMR). This
ensures removal of
lesions that are too
thick for ablative
therapy and may detect
HGD or occult cancers.
In order to allow
healing, at least 8
weeks should be allowed
between EMR and RFA. In
cases of HGD, endoscopic
ultrasound (EUS) may be
of value in ruling out
submucosal or lymph node
involvement.
Inclusion Criteria
Patients who satisfy the
following criteria may
be eligible for RFA
treatment
1. Age 18 to 80 years.
2. Patients who have
documented IM with
dysplasia.
In patients with low
grade dysplasia the
diagnosis should have
been documented within
the previous 12 months
and whilst receiving a
PPI.
In patients with high
grade dysplasia the
mucosa should have a
regular, non-nodular and
non-ulcerated appearance
and the diagnosis should
have been documented
within the previous 6
months and whilst
receiving a PPI.
In both low grade and
high-grade dysplasia the
slides should have been
reviewed by a second
expert gastrointestinal
pathologist.
3. Patient should be
able to take a PPI.
4. The patient should be
available for treatment
and follow-up endoscopy
as per the algorithm.
Exclusion criteria
1. The patient is
pregnant or planning a
pregnancy during the
course of RFA.
2. The presence of an
oesophageal stricture,
which prevents the
passage of the endoscope
or catheter.
3. Active oesophagitis
(Los Angeles
Classification Grade B
or higher).
4. Prior radiation
therapy to the
oesophagus. Radiation
therapy to the head and
neck region is not a
contra-indication to
RFA.
5. A history of
endoscopic mucosal
resection (EMR) that
meets any of the
following criteria
EMR performed less than
8 weeks before the RFA
session
EMR performed in a wide
field manner
(encompassing more than
90º of any area of the
oesophagus).
6. Any previous
oesophageal surgery
except fundoplication
without compli-cation
(i.e. slippage or
dysphagia).
7. The presence of
oesophageal varices.
8. The presence of an
uncontrolled
coagulopathy (INR > 1.3
and/or a platelet count
< 75,000/μL)
9. The presence of
comorbidities with an
anticipated life
expectancy of less than
2 years.
10. A known history of
alcohol and/or drug
dependency that would
limit the patient’s
ability provide informed
consent, to follow
post-treatment
instructions or to keep
follow-up appointments.
11. The patient has an
implanted pacing device
such as a cardiac
pacemaker, AICD or
neurostimulator and has
not received clearance
for RFA by the
specialist responsible
for the pacing device.
12. The patient suffers
from psychiatric or
other illness, which
makes compliance
unlikely.
SAGES
Responsibilities
Site Selection
SAGES will select a
number of medical
centres throughout South
Africa to initiate the
RFA Programme. The
centres will be selected
on the basis of a
demonstrated interest BO
and oesophageal cancer,
expertise in performing
advanced diagnostic and
therapeutic
interventions and the
availability of at least
2 expert
gastrointes-tinal
pathologists with an
interest in BO. Sites in
Pretoria, Johannesburg,
Durban, Bloemfontein and
Cape Town are under
consideration.
Training
Two gastroenterologists
at each centre will be
trained in RFA
techniques. Each should
have demonstrated
expertise in advanced
therapeutic
interventions such as
EMR, endoscopic
coagulation techniques
(argon plasma
coagulation, clipping)
and oesophageal
pneumatic dilatation.
Training will consist of
didactic and practical
components and will
initially be conducted
by a professional from
the manufacturer or one
its appointees. It is
foreseen that this
function will be taken
over by South African
endoscopists once a core
group has been trained
and developed the
necessary expertise.
SAGES furthermore
intends to provide
ongoing education in the
form of lectures and
live case
demonstrations.
Proposed lecture
material will include
the following topics
1. Endoscopic detection
techniques and
macroscopic
classification.
2. The role of and EUS
and EMR.
3. Technical aspects of
RFA.
4. Patient selection.
5. Procedural steps.
6. Post-ablation care.
Monitoring
SAGES will visit
participating centres to
evaluate the programme
and will require regular
reports on the outcomes
of the RFA program.
Patient Education
Patient education
remains the
responsibility of the
treating physician but
SAGES intends to assist
this process by
developing suitable
educational material.
15/11/2009 22:43